How Do Modern Scientists Describe The Makeup Of Matter
1.three: The Scientific Arroyo to Cognition
- Page ID
- 25455
Learning Objectives
- Give a brusque history of the concept of the cantlet.
- Describe the contributions of Democritus and Dalton to atomic theory.
- Summarize Dalton's atomic theory and explain its historical evolution.
We discussed the particulate model of thing, but this is not a clear conclusion to the casual observer of nature. While all modern scientists accept the concept of the cantlet, when the concept of the atom was first proposed most 2,500 years ago, ancient philosophers laughed at the thought. It has ever been difficult to convince people of the beingness of things that are too small to come across. Nosotros will spend some time considering the show (observations) that convince scientists of the beingness of atoms.
Virtually two,500 years ago, early Greek philosophers believed the entire universe was a single, huge, entity. In other words, "everything was one." They believed that all objects, all matter, and all substances were continued as a unmarried, large, unchangeable "thing." One of the first people to suggest "atoms" was a man known every bit Democritus. As an culling to the beliefs of the Greek philosophers, he suggested that atomos, or atomon - tiny, indivisible, solid objects - make up all matter in the universe.


Democritus then reasoned that changes occur when the many atomos in an object were reconnected or recombined in different ways. Democritus fifty-fifty extended this theory, suggesting that there were dissimilar varieties of atomos with unlike shapes, sizes, and masses. He idea, however, that shape, size, and mass were the simply properties differentiating the different types of atomos. Co-ordinate to Democritus, other characteristics, similar color and taste, did not reflect properties of the atomos themselves, but rather, resulted from the different means in which the atomos were combined and continued to one another.
The early Greek philosophers tried to understand the nature of the globe through reason and logic, but non through experiment and observation. Every bit a event, they had some very interesting ideas, but they felt no demand to justify their ideas based on life experiences. In a lot of means, you can think of the Greek philosophers every bit existence "all thought and no activeness." It's truly amazing how much they achieved using their minds, simply because they never performed any experiments, they missed or rejected a lot of discoveries that they could accept made otherwise. Greek philosophers dismissed Democritus' theory entirely. Sadly, it took over ii millennia earlier the theory of atomos (or "atoms," as they're known today) was fully appreciated.
Greeks: "All thought and No Action"
Greek philosophers were "all thought and no activeness" and did not feel the demand to test their theories with reality. In contrast, Dalton'southward efforts were based on experimentation and testing ideas against reality.
While it must be assumed that many more than scientists, philosophers, and others studied composition of matter after Democritus, a major jump forward in our understanding of the composition of matter took place in the 1800's with the work of the British scientists John Dalton. His diminutive theory is a fundamental concept that states that all elements are composed of atoms. Dalton formulated his theory by focusing on experimental results (in dissimilarity to the ancient Greek philosophers) past studied the weights of various elements and compounds. From his experiments and observations, likewise every bit the work from peers of his time, Dalton proposed a new theory of the atom.The general tenets of this theory were as follows:
- All matter is equanimous of extremely small particles called atoms.
- Atoms of a given chemical element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass, and other backdrop.
- Atoms cannot exist subdivided, created, or destroyed.
- Atoms of different elements can combine in unproblematic whole number ratios to form chemic compounds.
- In chemical reactions, atoms are combined, separated, or rearranged.
Dalton'south diminutive theory has been largely accustomed by the scientific community, with the exception of iii changes. Nosotros know at present that (1) an atom can be further subdivided, (2) all atoms of an element are non identical in mass, and (3) using nuclear fission and fusion techniques, we tin can create or destroy atoms by irresolute them into other atoms.
The bear witness for atoms is so great that few doubt their existence. In fact, private atoms are now routinely observed with state-of-the art technologies.
The Scientific Method
Scientists search for answers to questions and solutions to problems by using a process chosen the scientific method . This procedure consists of making observations, formulating hypotheses, and designing experiments, which in turn lead to additional observations, hypotheses, and experiments in repeated cycles (Figure \(\PageIndex{ii}\)).
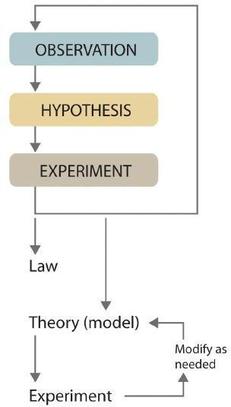
Observations can be qualitative or quantitative. Qualitative observations describe backdrop or occurrences in means that practise not rely on numbers. Examples of qualitative observations include the following: the outside air temperature is libation during the wintertime season, tabular array salt is a crystalline solid, sulfur crystals are yellow, and dissolving a penny in dilute nitric acid forms a blue solution acid forms a blue solution and a chocolate-brown gas. After deciding to learn more about an observation or a prepare of observations, scientists generally brainstorm an investigation by forming a hypothesis , a tentative explanation for the ascertainment(south). The hypothesis may not be correct, only it puts the scientist'southward understanding of the organization being studied into a class that can be tested. After a hypothesis has been formed, scientists behave experiments to test its validity. Experiments are systematic observations or measurements, preferably made under controlled conditions—that is, under conditions in which a single variable changes. For example, in the dinosaur extinction scenario, iridium concentrations in the dinosaur extinction scenario, iridium concentrations were measured worldwide and compared. A properly designed and executed experiment enables a scientist to determine whether the original hypothesis is valid. Experiments often demonstrate that the hypothesis is incorrect or that it must be modified. More experimental data are then collected and analyzed, at which betoken a scientist may begin to think that the results are sufficiently reproducible (i.east., dependable) to merit being summarized in a law , a exact or mathematical description of a phenomenon that allows for general predictions. A law simply says what happens; information technology does not accost the question of why.
It is important to remember that scientists take a tendency to formulate hypotheses in familiar terms merely because it is hard to advise something that has never been encountered or imagined before. As a upshot, scientists sometimes disbelieve or overlook unexpected findings that disagree with the basic assumptions backside the hypothesis or theory being tested. Fortunately, truly of import findings are immediately subject area to independent verification by scientists in other laboratories, so science is a self-correcting discipline.
Fundamental Definitions in Chemistry: https://youtu.be/SBwjbkFNkdw
Measurementsunderlie the Scientific Method
The results of a scientific experiment must be communicated to be of value. This affords an opportunity for other scientists to check them. Information technology as well allows the scientific community, and sometimes the full general public, to share new noesis. Advice, however, is not ever as straightforward as it might seem. Ambiguous terminology can often turn a seemingly clear statement into a morass of misunderstanding.
As an example, consider someone tells you that the forecast is a high of 25°. Do you put on a winter jacket, or summertime wear? If you are thinking winter, and so you interpreted the temperature equally 25°F. Yet if 25°C was meant, which is equal to 77°F, a winter jacket would exist far too warm. Or consider filling upward a car with gasoline. If yous are in the U.s.a., you will exist dealing in dollars per gallon, whereas, if you were in continental Europe, you will exist dealing in Euros per liter. Given that the exchange rate from USD to Euros fluctuates and that at that place are roughly 3.79 liters in i gallon, it is hard to simply compare numbers betwixt gas prices in the U.s., and say, France, if you don't know what units you are using. As a concluding example, consider the speed 24 meters/sec. Do you lot interpret this every bit close to highway speed limits in the United states of america? It is the same speed as 53.vii miles per 60 minutes, likely a more familiar set of units for speed for people in the US.
Scientists are non all that different from other people—they likewise have favorite units which are especially suited to sure areas of research. Still, scientists have constantly pressed for improvement and uniformity in systems of measurement. The first such activeness occurred virtually 200 years agone when, in the aftermath of the French Revolution, the metric system spread over most of continental Europe and was adopted by scientists everywhere. The United States nearly followed suit, but in 1799 Thomas Jefferson was unsuccessful in persuading Congress that a system based on powers of 10 was far more convenient and would eventually become the standard of the world.
The metric organisation has undergone continuous evolution and comeback since its original adoption by French republic. Offset in 1899, a series of international conferences accept been held for the purpose of redefining and regularizing the system of units. In 1960 the Eleventh Conference on Weights and Measures proposed major changes in the metric organisation and suggested a new name — the International Organisation of Units — for the revised metric arrangement. (The abbreviation SI , from the French Système International, is commonly used.) Scientific bodies such as the U.Due south. National Bureau of Standards and the International Union of Pure and Practical Chemical science have endorsed the SI.
Summary
- Chemists expand their knowledge by making observations, carrying out experiments, and testing hypotheses to develop laws to summarize their results and theories to explain them. In doing so, they are using the scientific method.
- 2,500 years ago, Democritus suggested that all thing in the universe was made up of tiny, indivisible, solid objects he called "atomos." Nonetheless, other Greek philosophers disliked Democritus' "atomos" theory considering they felt it was illogical. Dalton'southward Atomic Theory is the kickoff scientific theory to chronicle chemical changes to the structure, properties, and behavior of the cantlet.
- The natural sciences brainstorm with ascertainment , and this usually involves numerical measurements of quantities such every bit length, volume, density, and temperature. Well-nigh of these quantities have units of some kind associated with them, and these units must be retained when you employ them in calculations. Measuring units can be defined in terms of a very small number of primal ones that, through "dimensional analysis", provide insight into their derivation and meaning, and must exist understood when converting betwixt different unit systems.
Contributions & Attributions
This page was constructed from content via the following correspondent(s) and edited (topically or extensively) past the LibreTexts development team to meet platform style, presentation, and quality:
How Do Modern Scientists Describe The Makeup Of Matter,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Structure_and_Properties_(Tro)/01%3A_Atoms/1.03%3A_The_Scientific_Approach_to_Knowledge
Posted by: grillomurds1936.blogspot.com


0 Response to "How Do Modern Scientists Describe The Makeup Of Matter"
Post a Comment